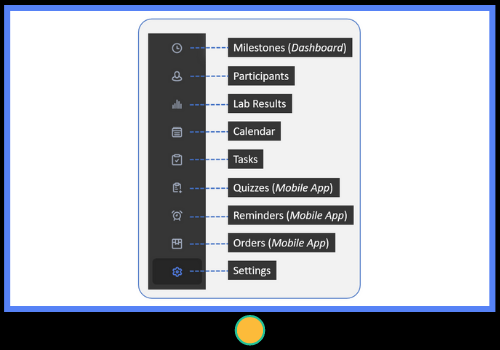
- Use SMaRT with a prospective cohort, randomized controlled trial (RCT), cross-sectional, or qualitative study design
- Accommodate study staff and participants involved in multiple studies
- Specify study details, milestones for each arm or treatment group, site specific email addresses, automated notifications (email/text/push), and custom fields
- Create visit activities, task activities, or survey activities with custom URLs and survey completion feedback
- Insert ad hoc activities as needed on individual participant timelines
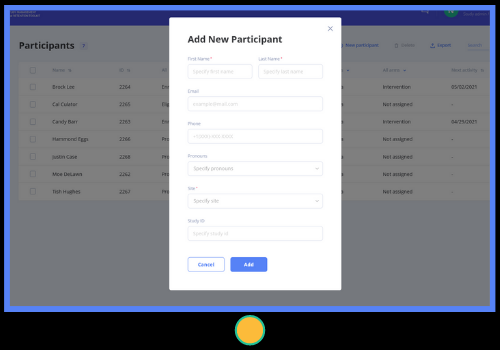
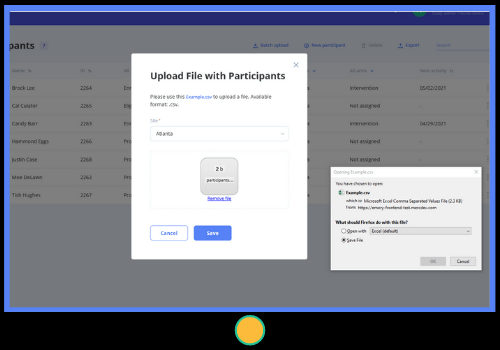
- Enter participants manually or import in bulk
next feature ↳
↵ back to full list of features